kidzsearch.com >wiki Explore:webimagesvideosgames
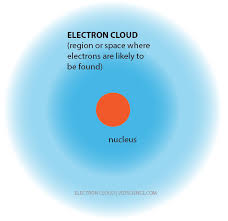
An electron cloud is the area where chance of electron presence in -surrounding to an atomic nucleus is maximum. It is representing a region in which maximum probability of e. Electron cloud defines the zone of probability describing the electron’s location, because of the uncertainty principle.In the electron cloud model, the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Electron cloud model is a model of an atom, in which the atom consist of a small but massive nucleus. An electron cloud is the region of negative charge surrounding an atomic nucleus that is associated with an atomic orbital. It is defined mathematically, describing a region with a high probability of containing electrons. The modern model is also commonly called the electron cloud model. That’s because each orbital around the nucleus of the atom resembles a fuzzy cloud around the nucleus, like the ones shown in the Figure below for a helium atom. The densest area of the cloud is where the electrons have the greatest chances of.
Electron Cloud Gif
Electron cloud is an informal way to describe an atomic orbital.
The electron cloud is not really a thing. An electron cloud model is different from the older Bohr atomic model by Niels Bohr. Bohr talked about electrons orbiting the nucleus. Explaining the behavior of these electron 'orbits' was a key issue in the development of quantum mechanics.[1]
The electron cloud model says that we cannot know exactly where an electron is at any given time, but the electrons are more likely to be in specific areas. These areas are specified by orbitals. The orbitals are specified by shells and sub-orbitals. In the Bohr model, electrons are assigned to different shells. The shells, k,l,m,n,o,p,q, each represent different levels of energy, and are also called energy levels. The sub-orbitals; s,p,d,f, are regions where it will be more likely to find electrons, and can each hold a different number of electrons. the s,p,d,f orbitals are all shaped differently. This can be proven by the repeating patterns of chemical properties in the periodic table. Using quantum mechanics, chemists can use the electron cloud model to assign electrons to different atomic orbitals. Atomic orbitals also explain the patterns in the periodic table.
The electron cloud model was developed in 1926 by Erwin Schrödinger and Werner Heisenberg. The model is a way to help visualize the most probable position of electrons in an atom. The electron cloud model is currently the accepted model of an atom.

According to Bohr's calculations for a hydrogen atom, the electron under normal conditions always stays at a certain distance from the nucleus. This distance is called the Bohr radius and is approximately 0.529 Å (0.529×10−10 m). But according to the wave mechanical or cloud concept model, the electron keeps on moving away or towards the nucleus and the maximum probability of locating it lies at a distance of 0.529 Å from the nucleus. In other words, the radius of the electron cloud or the radius of maximum probability is 0.529 Å.
References
- ↑Bryson, Bill 2003. A short history of nearly everything. Broadway Books. pp. 141–143. ISBN0-7679-0818-X.
Electron Cloud Model Definition
Cloud Model Of Atom
