Chemical properties of magnesium - Health effects of magnesium - Environmental effects of magnesium
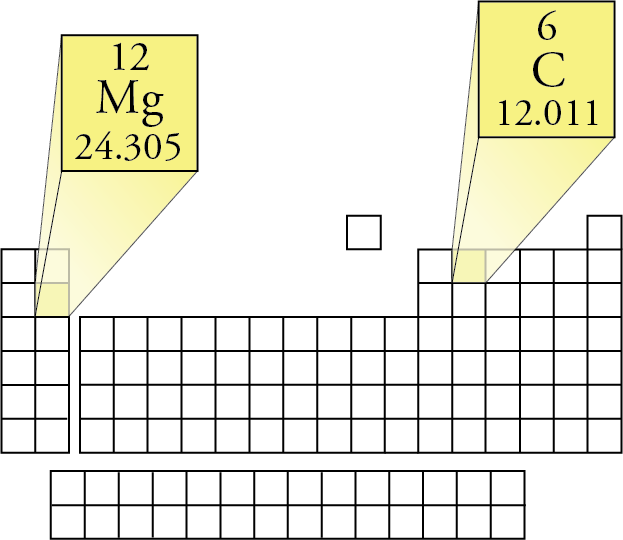
- 01 Magnesium symbol: Mg Atomic mass; 24.305 u Atomic number: 12 Electron configuration: Ne 3s2 Melting point: 650 °C boiling point: 1107 °C A T O M I C E L E M E N T Magnesium is utilized in items that profit by being lightweight, for example, vehicle seats, baggage, laptops, cameras, and power tools.
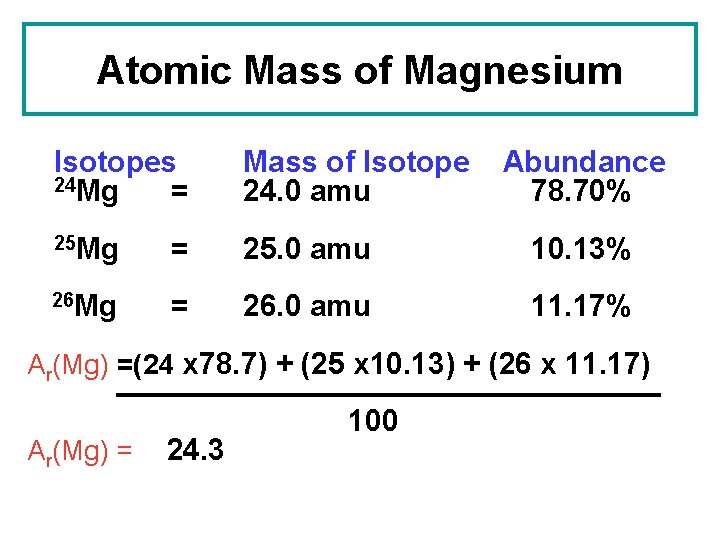
- Atomic mass (Da) Isotopic abundance (amount fraction) 24 Mg: 23.985 041 70(9) 0.7888, 0.7905 25 Mg: 24.985 8370(3) 0.099 88, 0.100 34 26 Mg: 25.982 5930(2) 0.1096, 0.1109 In 2011, the Commission has changed the standard atomic weight of magnesium to A r (Mg) = 24.304, 24.307 based on an evaluation of the effect of variation in isotopic.
Magnesium is a very light metal. Only calcium and the alkali metals are lighter. This low density is used to advantage in alloys like magnalium (10% magnesium, 90% aluminum). Magnesium reacts with boiling water to form magnesium hydroxide and hydrogen gas. The metal burns in air with a bright white light, leading to application in flares.
|
MagnesiumChemical element, metallic, symbol Mg, situated in group IIa in the periodic table, atomic number: 12, atomic weight: 24,312. Magnesium is silvery white and very light. Its relative density is 1,74 and it’s density 1740 kg/m3 (0.063 lb/in3 or 108.6 lb/ft3). Magnesium is known for a long time as the lighter structural metal in the industry, due to it’s low weight and to it’s capability of forming mechanically resistant alloys. Magnesium is very chemically active, it takes the place of hydrogen in boiling water and a great number of metals can be produced by thermic reduction of its salts and oxidized forms with magnesium. It joins together with most non-metals and almost every acid. Magnesium reacts only slightly or not at all with most of the alkalis and many organic substances, like hydrocarbons, aldehides, alcohols, phenols, amines, esters and most of the oils. Used as a catalyst, magnesium promotes organic reactions of condensation, reduction, addition and dehalogenization. It was used for a long time for synthesizing special and complex organic components by the well-known Grignard reaction. The main ingredients of the alloys are: aluminum, manganese, zircon, zinc, rare-earth metals and thorium. Applications Magnesium compounds are used as refractory material in furnace linings for producing metals (iron and steel, nonferrous metals), glass, and cement. Magnesium in the environment Magnesium is the eighth most abundant element and constitutes about 2% af the Earth's crust by weight, and it is the third most plentiful element dissolved in seawater. Health effects of magnesium
There is no evidence that magnesium produces systemic poisoning although persistent over-indulgence in taking magnesium supplements and medicines can lead to muscule weakness, lethargy and confusion. Effects of exposure to magnesium powder: low toxicity & not considered to be hazardous to health. Inhalation: dust may irritate mucous membranes or upper respiratory tract. Eyes: mechanical injury or particle may embed in eye. Viewing of burning magnesium powder without fire glasses may result in 'Welder's flash', due to intense white flame. Skin: embedding of particle in skin. Ingestion: unlikely; however, ingestion of large amounts of magnesium powder could cause injury. Physical dangers:Dust explosion possible if in powder or granular form, mixed with air. If dry, it can be charged electrostatically by swirling, pneumatic transport, pouring, etc. First Aid: Inhalation: remove to fresh air. Eyes: flush eyes with water thoroughly. Consult a physician. Skin: wash with soap & water thoroughly to remove particles. Ingestion: if large amounts of magnesium powder are ingested, induce vomiting & consult a physician. Note to physician: no specific treatment or antidote. Supportive care recommended. Treatment should be based on reactions of the patient.
|
More from 'Elements'
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Mg Atomic Number
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Mg(oh)2 Atomic Mass
Copyright © 1998-2021 Lenntech B.V. All rights reserved